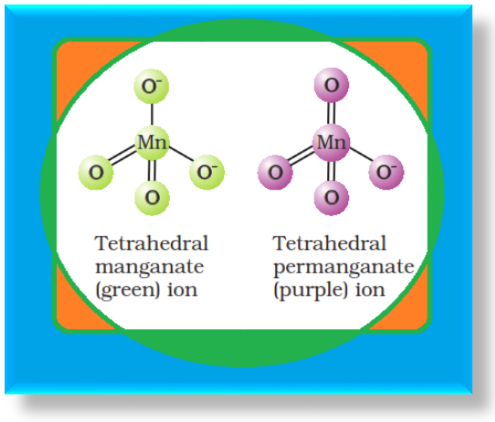
Potassium Dichromate (`K_2Cr_2O_7`) :
`=>` Potassium dichromate is a very important chemical used in leather industry and as an oxidant for preparation of many azo compounds.
`color{green}(text(Preparation ))` : Dichromates are generally prepared from chromate, which in turn are obtained by the fusion of chromite ore (`color{red}(FeCr_2O_4)`) with sodium or potassium carbonate in free access of air.
● The reaction with sodium carbonate occurs as follows :
`color{red}(4 FeCr_2O_4 + 8 Na_2CO_3 + 7 O_2 → 8 Na_2 CrO_4 + 2 Fe_2 O_3 + 8 CO_2)`
● The yellow solution of sodium chromate is filtered and acidified with sulphuric acid to give a solution from which orange sodium dichromate, `color{red}(Na_2Cr_2O_7. 2H_2O)` can be crystallised.
`color{red}(2Na_2CrO_4 + 2 H^(+) → Na_2Cr_2O_7 + 2 Na^(+) + H_2O)`
● Sodium dichromate is more soluble than potassium dichromate. The latter is therefore, prepared by treating the solution of sodium
dichromate with potassium chloride.
`color{red}(Na_2Cr_2O_7 + 2 KCl → K_2Cr_2O_7 + 2 NaCl)`
● Orange crystals of potassium dichromate crystallise out.
`=>` The chromates and dichromates are interconvertible in aqueous solution depending upon `pH` of the solution.
`=>` The oxidation state of chromium in chromate and dichromate is the same.
`color{red}(2 CrO_4^(2–) + 2H^(+) → Cr_2O_7^(2–) + H_2O)`
`color{red}(Cr_2O_7^(2–) + 2 OH^(-) → 2 CrO_4^(2–) + H_2O)`
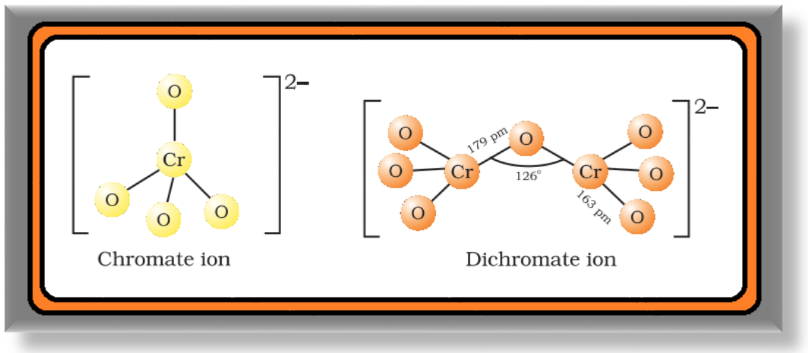
`=>` The structures of chromate ion, `color{red}(CrO_4^(2-))` and the dichromate ion, `color{red}(Cr_2O_7^(2-))` are shown below.
● The chromate ion is tetrahedral whereas the dichromate ion consists of two tetrahedra sharing one corner with `color{red}(Cr–O–Cr)` bond angle of `126°`.
`color{green}(text(Properties ))` : Sodium and potassium dichromates are strong oxidising agents; the sodium salt has a greater solubility in water and is extensively used as an oxidising agent in organic chemistry.
`=>` Potassium dichromate is used as a primary standard in volumetric analysis.
● In acidic solution, its oxidising action can be represented as follows :
`color{red}(Cr_2O_7^(2–) + 14H^(+) + 6e → 2Cr^(3+) + 7H_2O \ \ \ \ (EV = 1.33V))`
● Thus, acidified potassium dichromate will oxidise iodides to iodine, sulphides to sulphur, tin(II) to tin(IV) and iron(II) salts to iron(III). The half-reactions are noted below :
`color{red}(6 I^(–) → 3I_2 + 6 e^(–)`; `3 Sn^(2+) → 3Sn^(4+) + 6 e^–)`
`color{red}(3 H_2 S → 6H^(+) + 3S + 6e^(–)`; `6 Fe^(2+) → 6Fe^(3+) + 6 e^–)`
● The full ionic equation may be obtained by adding the half-reaction for potassium dichromate to the half-reaction for the reducing agent, for e.g.,
`color{red}(Cr_2O_7^(2–) + 14 H^(+) + 6 Fe^(2+) → 2 Cr^(3+) + 6 Fe^(3+) + 7 H_2O)`
`color{green}(text(Preparation ))` : Dichromates are generally prepared from chromate, which in turn are obtained by the fusion of chromite ore (`color{red}(FeCr_2O_4)`) with sodium or potassium carbonate in free access of air.
● The reaction with sodium carbonate occurs as follows :
`color{red}(4 FeCr_2O_4 + 8 Na_2CO_3 + 7 O_2 → 8 Na_2 CrO_4 + 2 Fe_2 O_3 + 8 CO_2)`
● The yellow solution of sodium chromate is filtered and acidified with sulphuric acid to give a solution from which orange sodium dichromate, `color{red}(Na_2Cr_2O_7. 2H_2O)` can be crystallised.
`color{red}(2Na_2CrO_4 + 2 H^(+) → Na_2Cr_2O_7 + 2 Na^(+) + H_2O)`
● Sodium dichromate is more soluble than potassium dichromate. The latter is therefore, prepared by treating the solution of sodium
dichromate with potassium chloride.
`color{red}(Na_2Cr_2O_7 + 2 KCl → K_2Cr_2O_7 + 2 NaCl)`
● Orange crystals of potassium dichromate crystallise out.
`=>` The chromates and dichromates are interconvertible in aqueous solution depending upon `pH` of the solution.
`=>` The oxidation state of chromium in chromate and dichromate is the same.
`color{red}(2 CrO_4^(2–) + 2H^(+) → Cr_2O_7^(2–) + H_2O)`
`color{red}(Cr_2O_7^(2–) + 2 OH^(-) → 2 CrO_4^(2–) + H_2O)`
`=>` The structures of chromate ion, `color{red}(CrO_4^(2-))` and the dichromate ion, `color{red}(Cr_2O_7^(2-))` are shown below.
● The chromate ion is tetrahedral whereas the dichromate ion consists of two tetrahedra sharing one corner with `color{red}(Cr–O–Cr)` bond angle of `126°`.
`color{green}(text(Properties ))` : Sodium and potassium dichromates are strong oxidising agents; the sodium salt has a greater solubility in water and is extensively used as an oxidising agent in organic chemistry.
`=>` Potassium dichromate is used as a primary standard in volumetric analysis.
● In acidic solution, its oxidising action can be represented as follows :
`color{red}(Cr_2O_7^(2–) + 14H^(+) + 6e → 2Cr^(3+) + 7H_2O \ \ \ \ (EV = 1.33V))`
● Thus, acidified potassium dichromate will oxidise iodides to iodine, sulphides to sulphur, tin(II) to tin(IV) and iron(II) salts to iron(III). The half-reactions are noted below :
`color{red}(6 I^(–) → 3I_2 + 6 e^(–)`; `3 Sn^(2+) → 3Sn^(4+) + 6 e^–)`
`color{red}(3 H_2 S → 6H^(+) + 3S + 6e^(–)`; `6 Fe^(2+) → 6Fe^(3+) + 6 e^–)`
● The full ionic equation may be obtained by adding the half-reaction for potassium dichromate to the half-reaction for the reducing agent, for e.g.,
`color{red}(Cr_2O_7^(2–) + 14 H^(+) + 6 Fe^(2+) → 2 Cr^(3+) + 6 Fe^(3+) + 7 H_2O)`